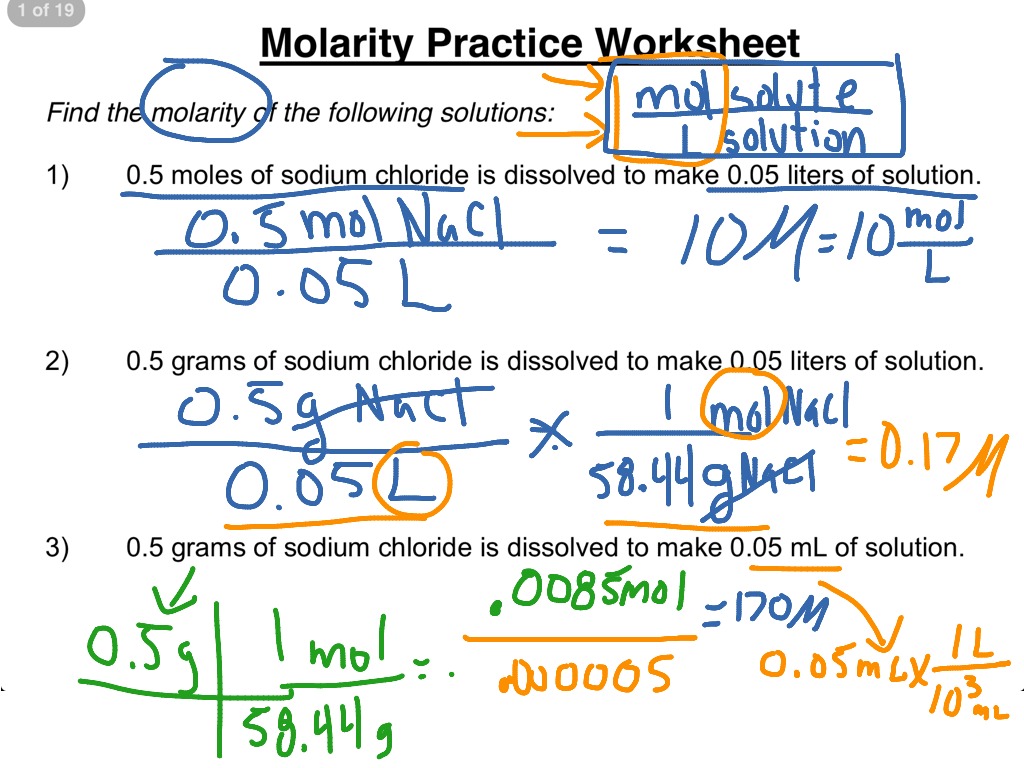
Dilutions And Molarity Worksheet . Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid. dilution problems worksheet 1. chemistry ii worksheet molarity, & dilution. Molality (m or ) = moles of solute kg of solvent. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Work each of the following problems in the space provided. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. molarity and dilutions worksheet. 2) if i add water to. 4.0 moles of licl is dissolved in 5.0 liters of water.
from worksheetfullunglued.z22.web.core.windows.net
1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. Work each of the following problems in the space provided. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilution problems worksheet 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? chemistry ii worksheet molarity, & dilution. 2) if i add water to. 4.0 moles of licl is dissolved in 5.0 liters of water. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid. molarity and dilutions worksheet.
Molarity Worksheet With Answers
Dilutions And Molarity Worksheet 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 4.0 moles of licl is dissolved in 5.0 liters of water. molarity and dilutions worksheet. 2) if i add water to. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Work each of the following problems in the space provided. chemistry ii worksheet molarity, & dilution. dilution problems worksheet 1. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. Molality (m or ) = moles of solute kg of solvent.
From worksheetfullunglued.z22.web.core.windows.net
Molarity Worksheet With Answers Dilutions And Molarity Worksheet Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid. chemistry ii worksheet molarity, & dilution. Molality (m or ) = moles of solute kg of solvent. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 2) if i add water to. 1). Dilutions And Molarity Worksheet.
From kidsworksheetfun.com
Molarity And Dilution Worksheet kidsworksheetfun Dilutions And Molarity Worksheet 4.0 moles of licl is dissolved in 5.0 liters of water. chemistry ii worksheet molarity, & dilution. molarity and dilutions worksheet. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. dilution problems. Dilutions And Molarity Worksheet.
From ataglance.randstad.com
Molarity And Dilution Worksheet Printable Calendars AT A GLANCE Dilutions And Molarity Worksheet Molality (m or ) = moles of solute kg of solvent. 2) if i add water to. chemistry ii worksheet molarity, & dilution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. molarity and dilutions worksheet. Calculate the final concentration of a solution. Dilutions And Molarity Worksheet.
From karenworksheetideas17paper.netlify.app
Molarity And Dilutions Worksheet Worksheet Educational Ideas Dilutions And Molarity Worksheet 2) if i add water to. Work each of the following problems in the space provided. chemistry ii worksheet molarity, & dilution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. molarity and dilutions worksheet. Calculate the final concentration of a solution that. Dilutions And Molarity Worksheet.
From studyschoolford.z21.web.core.windows.net
Molarity Dilution Practice Problems Dilutions And Molarity Worksheet Work each of the following problems in the space provided. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? dilution problems worksheet 1. 4.0 moles. Dilutions And Molarity Worksheet.
From lessonmagicnunez.z21.web.core.windows.net
Molarity And Dilution Worksheets Dilutions And Molarity Worksheet chemistry ii worksheet molarity, & dilution. 4.0 moles of licl is dissolved in 5.0 liters of water. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid. 1) if i add 25 ml of water. Dilutions And Molarity Worksheet.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Made By Teachers Dilutions And Molarity Worksheet Molality (m or ) = moles of solute kg of solvent. dilution problems worksheet 1. 4.0 moles of licl is dissolved in 5.0 liters of water. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid. Work each of the following problems in the space provided. 2) if i add water to. 1). Dilutions And Molarity Worksheet.
From www.scribd.com
Molarity and Dilution Worksheets PDF Molar Concentration Mole (Unit) Dilutions And Molarity Worksheet 4.0 moles of licl is dissolved in 5.0 liters of water. dilution problems worksheet 1. molarity and dilutions worksheet. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. 1) if i have 340 ml of a 0.5 m nabr solution, what will the. Dilutions And Molarity Worksheet.
From learninglibrarybayer.z13.web.core.windows.net
Molarity And Dilution Worksheet Dilutions And Molarity Worksheet Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid. chemistry ii worksheet molarity, & dilution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. 4.0 moles of licl is dissolved in 5.0 liters of water. 1) if. Dilutions And Molarity Worksheet.
From www.liveworksheets.com
Concentration gL, dilution, and molarity worksheet Live Worksheets Dilutions And Molarity Worksheet 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 2) if i add water to. dilution problems worksheet 1. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if i add. Dilutions And Molarity Worksheet.
From www.docsity.com
Molarity and Dilutions Practice Worksheet Key Docsity Dilutions And Molarity Worksheet Molality (m or ) = moles of solute kg of solvent. 4.0 moles of licl is dissolved in 5.0 liters of water. 2) if i add water to. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. dilution problems worksheet 1. molarity and. Dilutions And Molarity Worksheet.
From materialzonetomlin.z21.web.core.windows.net
Dilution Problems Worksheets Dilutions And Molarity Worksheet chemistry ii worksheet molarity, & dilution. Molality (m or ) = moles of solute kg of solvent. dilution problems worksheet 1. 4.0 moles of licl is dissolved in 5.0 liters of water. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution. Dilutions And Molarity Worksheet.
From www.madebyteachers.com
Dilution, Molarity, and Volume Calculations A Chemistry Worksheet Made By Teachers Dilutions And Molarity Worksheet Molality (m or ) = moles of solute kg of solvent. Work each of the following problems in the space provided. 4.0 moles of licl is dissolved in 5.0 liters of water. 2) if i add water to. dilution problems worksheet 1. chemistry ii worksheet molarity, & dilution. 1) if i have 340 ml of a 0.5 m. Dilutions And Molarity Worksheet.
From studydbderrico.z13.web.core.windows.net
Molarity And Dilution Worksheets Dilutions And Molarity Worksheet 2) if i add water to. Work each of the following problems in the space provided. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? chemistry ii worksheet molarity, & dilution. 4.0 moles of licl is dissolved in 5.0 liters of. Dilutions And Molarity Worksheet.
From lessonlibrarystower.z22.web.core.windows.net
Molarity Dilution Worksheet Dilutions And Molarity Worksheet molarity and dilutions worksheet. 4.0 moles of licl is dissolved in 5.0 liters of water. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid. dilution problems worksheet 1. 2) if i add water to. chemistry ii worksheet molarity, & dilution. Molality (m or ) = moles of solute kg of. Dilutions And Molarity Worksheet.
From worksheets.clipart-library.com
Molarity and Dilutions Worksheet Key Western Oregon University Worksheets Library Dilutions And Molarity Worksheet Molality (m or ) = moles of solute kg of solvent. Work each of the following problems in the space provided. 4.0 moles of licl is dissolved in 5.0 liters of water. 2) if i add water to. chemistry ii worksheet molarity, & dilution. dilution problems worksheet 1. Calculate the final concentration of a solution that is made. Dilutions And Molarity Worksheet.
From www.chegg.com
Solved Molarity And Dilutions Worksheet 1. What Is The Mo... Dilutions And Molarity Worksheet Molality (m or ) = moles of solute kg of solvent. dilution problems worksheet 1. molarity and dilutions worksheet. Work each of the following problems in the space provided. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? 1) if. Dilutions And Molarity Worksheet.
From karenworksheetideas17paper.netlify.app
Molarity And Dilutions Worksheet Worksheet Educational Ideas Dilutions And Molarity Worksheet Work each of the following problems in the space provided. 1) if i have 340 ml of a 0.5 m nabr solution, what will the concentration be if. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted. 4.0 moles of licl is dissolved in 5.0. Dilutions And Molarity Worksheet.